This article was produced in partnership with the Gothamist.
In a new study, Columbia University researchers have identified coronavirus mutations in New York City wastewater that seem to appear when severe disease rates begin to rise. The findings may pinpoint subtle, understated variants in the pandemic that are affecting day-to-day outcomes, including hospitalization and death, without doctors noticing.
“Wastewater is a pooled sample,” said Archana Anand, a postdoctoral researcher at Columbia who worked on the study, which includes data from fall 2020 through winter 2022. By collecting genetic information from these wastewater samples, researchers can study the virus strains circulating among hundreds of thousands of people at once, rather than gathering this information one PCR test swab at a time.
To identify potential leads on what those novel mutations do, Anand linked the wastewater data with data on cases, hospitalizations and deaths from the New York City and New Jersey health departments. By connecting COVID-19 patterns in ZIP codes served by the north Manhattan plant and in Bergen County to the mutations present in these sewersheds, she found some mutations that may be helping the coronavirus move faster and evade people’s immune systems.
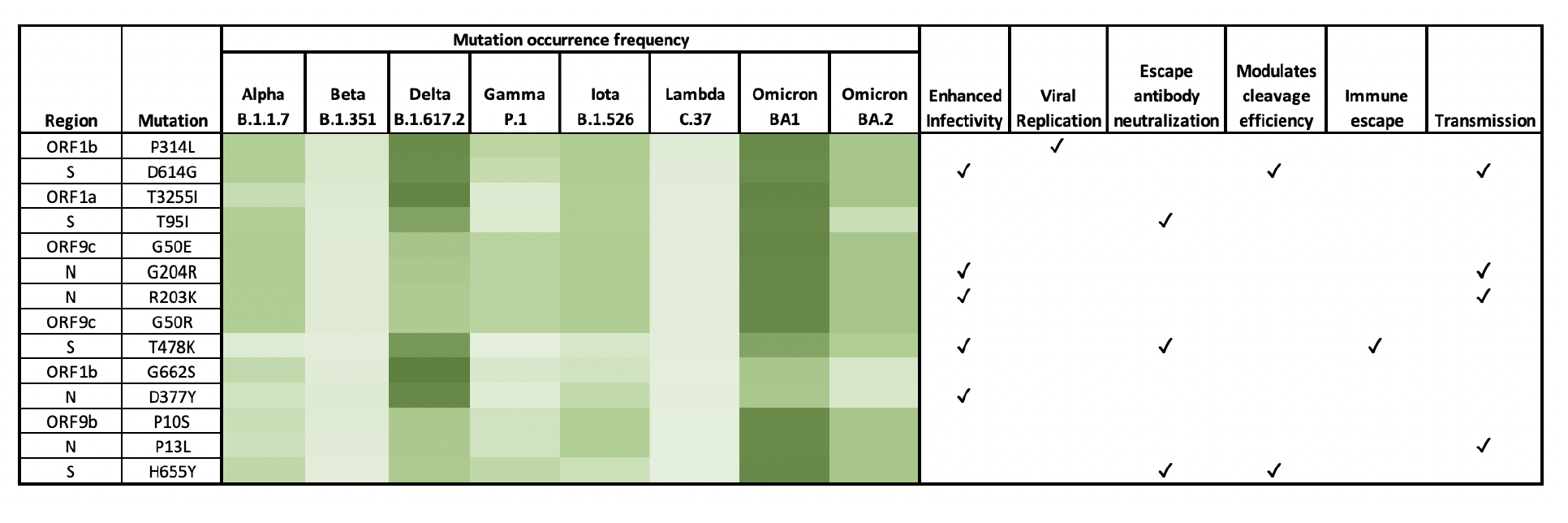
"Analysis of SARS-CoV-2 amino acid mutations in New York City Metropolitan wastewater (2020-2022) reveals multiple traits with human health implications across the genome and environment-specific distinctions" by Anand Archana, Chenghua Long and Kartik Chandran
For example, she identified three mutations on the N protein — a piece of the virus that protects its genetic material, along with serving other functions. When these mutations were present in the wastewater, hospitalization, mortality and test positivity rates were higher.
The Columbia team is led by engineering professor and wastewater expert Kartik Chandran. Prior to the pandemic, Chandran tracked microbial communities in wastewater, leading his lab to easily pivot to the coronavirus.
Most wastewater surveillance in the U.S. focuses on measuring how much coronavirus is present in a given sewershed. But Chandran is interested in answering more complex questions.
“It’s not just the ‘who’ and ‘how many,’” he said. “It’s the function and activity.”
In other words: not just how much coronavirus is in the sewage, but which variants, and what those variants are capable of.
Tracking variants through wastewater rather than PCR tests is “more comprehensive,” said Marc Johnson, a professor of microbiology and immunology at the University of Missouri who leads the state’s wastewater surveillance program, and was not involved with the Columbia study.
“It tells you about everyone,” he said, including people “who don’t even know they’re sick.” Wastewater surveillance has become more widespread over the past year, thanks in part to funding from the Centers for Disease Control and Prevention. But setting up sewage testing — and incorporating variant tracking — can be logistically difficult for health departments.
Anand and her colleagues flagged specific coronavirus mutations that they say deserve further attention as potential drivers of increased transmission or severity. The study, which has not yet been peer-reviewed, may be a starting point for identifying the next major variant of concern in New York City, or even for designing variant-specific treatments and vaccines.
The Documenting COVID-19 project, which partnered with Gothamist to publish this article, explored this study’s implications through interviews with its authors and outside experts, as part of its ongoing research into wastewater surveillance for COVID-19. The Documenting COVID-19 project is partially supported by Columbia University’s Brown Institute for Media Innovation, which is not affiliated with the lab who conducted this study.
Identifying new mutations
Chandran’s team analyzes wastewater samples from Columbia University buildings as well as two wastewater treatment plants: the North River Wastewater Treatment Plant in upper Manhattan and the Little Ferry Wastewater Treatment Plant across the river in Bergen County, New Jersey. Each plant serves about half a million people.
By sequencing these samples, the researchers could track the different variants New Yorkers have become all too acquainted with over the last year and a half. Alpha and iota (the “New York variant”) dominated sewersheds in early 2021, followed by delta and omicron.
Johnson recognized many of the mutations flagged in the study as “part of variants of concern,” which are considered more detrimental by public health agencies.
Chandran and his team also went beyond identifying these variants by analyzing all parts of the coronavirus genome. Much research has focused on the coronavirus spike protein, the part that attaches to human cells and enables its entry.
Given the spike protein’s vital role, it’s arguably the most well-studied part of the coronavirus. Our immune systems also try to block the spike, creating selective pressure that forces the virus to adapt. More mutation happens in the spike protein than any other part of the virus, Johnson said.
But other segments of the coronavirus that are less studied could play roles in how it spreads.
“We were never going to restrict ourselves to the spike protein,” Chandran said. “It’s looking at one single biomarker, like looking at cholesterol and blaming it for heart disease.”
Finding function
About 60% of mutations flagged in the new study happen in parts of the virus outside the spike protein, Anand said. In addition, her analysis found “several mutations for which we do not understand their functional implications yet.”
More research is needed to solidify these connections. It can be difficult to tie a specific mutation to a specific viral quality. That’s typically done in a lab — in a petri dish or animal model of the disease.
“With any mutation, it’s important to first discount the possibility that its prevalence is not due to it being ‘carried along’ by a different mutation,” said Shishi Luo, associate director of bioinformatics and infectious diseases at the variant-tracking company Helix. In these situations, two mutations might show up in an alarming variant because they happened to evolve together, but only one may actually give the virus a meaningful advantage.
The Columbia study did not include animal models that would actively test which mutations are dangerous and which are simply carried along. Chandran and Anand hope that other researchers will follow up on their work with this analysis.
New York City is a prime location for tracking the coronavirus through wastewater because it’s so large and dense, Johnson said. Researchers can essentially test millions of people with only a few collected samples. But other places should also do this work, he added, “because you never know where something new is gonna happen.”
Luo hopes to see variants tracked — and linked to other types of data — across the country.
“If the specific analysis in this preprint can be implemented in real time,” she said, “it would be a great first step towards an early warning system.”
Such an alert system could both flag new mutations and determine how they might change the pandemic’s severity.




